Propargylation of 1,3-dicarbonyl compounds catalyzed by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone and sodium nitrite in the presence of molecular oxygen and formic acid†
Abstract
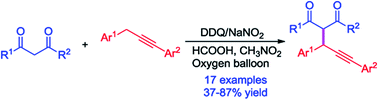
Catalyzed by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and sodium nitrite, the coupling reaction of 1,3-diarylpropynes and 1,3-dicarbonyl compounds using molecular oxygen as a terminal oxidant was developed. The reaction was promoted dramatically in the presence of HCOOH. The corresponding products were obtained within half an hour in 37–87% yields.

 Please wait while we load your content...
Please wait while we load your content...