A combined experimental–theoretical study of the acid–base behavior of mangiferin: implications for its antioxidant activity†
Abstract
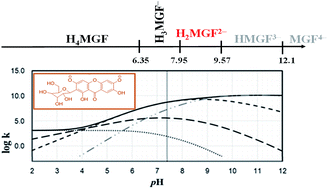
Acidity constants of mangiferin (H4MGF) in DMSO/H2O (80%/20%, v/v) were determined by UV-Visible and 1H and 13C NMR spectroscopies. UV-Visible absorption spectra in the 4.2 ≤ pH ≤ 11.7 range were fitted using the computational program SQUAD, for the refining of pKa values, obtaining as results: pKa1 = 7.337 ± 0.001, pKa2 = 8.936 ± 0.001, and pKa3 = 10.297 ± 0.028. The sigmoidal curves of the chemical shifts of 1H and 13C in a similar pH range were fitted using the same model refined by SQUAD for UV-Visible data. The behavior of the chemical shifts as a function of pH allowed us to assign the deprotonation order. A theoretical DFT study has been followed confirming the deprotonation order determined experimentally, as well as a proposed mechanism of antioxidant activity, that considers the fractions of the different species at physiological pH. It was found that mangiferin is an excellent peroxyl radical scavenger in aqueous solution, significantly surpassing the activity of Trolox, which is a well-known antioxidant frequently used as a reference in this context.


 Please wait while we load your content...
Please wait while we load your content...