Asymmetric tandem hemiaminal-heterocyclization-aza-Mannich reaction of 2-formylbenzonitriles and amines using chiral phase transfer catalysis: an experimental and theoretical study†
Abstract
The first asymmetric synthesis of 3-amino-substituted isoindolinones was accomplished via cascade hemiaminal-heterocyclization-intramolecular aza-Mannich reaction of benzylamines and 2-formylbenzonitriles using chiral phase transfer conditions (PTC). A theoretical study of the enantioselective step provides a rationale for the mode of action of the best performing phase transfer catalyst and the observed face selectivity.
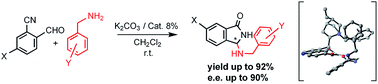

 Please wait while we load your content...
Please wait while we load your content...