Phosphine-free chiral iridium catalysts for asymmetric catalytic hydrogenation of simple ketones†
Abstract
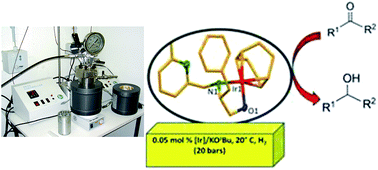
Novel pyridylalkylamine and aminopyridinato ligand stabilized iridium complexes with no P ligand are introduced. These complexes have been investigated as catalysts for asymmetric hydrogenation of simple ketones, resulting in an active catalyst for bulky alkyl aryl ketones that is α-methylpropiophenone. The ligands were synthesized from inexpensive starting materials and their modular design allows for the introduction of a broad variety of substitution patterns. Additionally, better activity and selectivity was observed at 20 °C and 20 bar H2 pressure with a catalyst loading as low as 0.05 mol% iridium. These phosphorus free catalysts have always been a central issue in both academic and industrial research.


 Please wait while we load your content...
Please wait while we load your content...