Electrocatalytic study of a 1,10-phenanthroline–cobalt(ii) metal complex catalyst supported on reduced graphene oxide towards oxygen reduction reaction†
Abstract
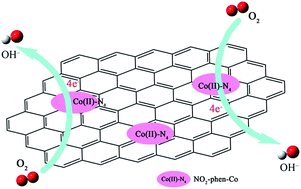
A new class of oxygen reduction reaction (ORR) catalyst was fabricated by loading a 1,10-phenanthroline–cobalt(II) metal-complex onto reduced graphene oxide (rGO) surfaces by π–π interaction. The Co(II)–N4 was the active center of the metal-complex catalyst and catalyzed the ORR via a highly efficient four-electron reduction pathway. The introduction of the nitro-group substituent in 1,10-phenanthroline highly boosted the catalytic activity of the metal complex in terms of half-wave potential (E1/2) and kinetic current density (JK), due to the downshift of the eg-orbital energy level for central Co(II) resulting from the electron-withdrawing effect of the nitro group. Considering the configuration of the metal complex on rGO surfaces, a single cobalt center-mediated catalytic mechanism was proposed to elucidate the ORR process. Compared with the commercial Pt/C catalyst, the as-prepared metal-complex catalyst exhibited a superior methanol tolerance and catalytic durability for the ORR. Our study provides more information about the relationship between the molecular structure and catalytic activity towards the ORR.

 Please wait while we load your content...
Please wait while we load your content...