Microwave-assisted one-pot synthesis of steroid–quinoline hybrids and an evaluation of their antiproliferative activities on gynecological cancer cell lines†
Abstract
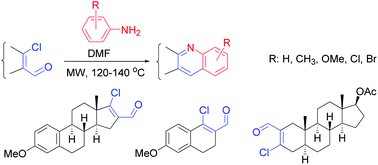
Novel D- and A-ring-fused quinolines in the estrone and 5α-androstane series were efficiently synthesized from the corresponding β-chlorovinyl aldehydes with different arylamines in DMF under microwave irradiation. The rates of the one-pot catalyst-free syntheses and the yields of the desired products were found to be affected significantly by the electronic and steric character of the substituents on the anilines and the different reactivities of rings D and A of the sterane skeleton. All the synthesized compounds were tested in vitro on human cervical (C33A, HeLa and SiHa) and breast (MCF-7, MDA-MB-231, MDA-MB-361 and T47D) cancer cell lines in order to investigate their antiproliferative activities in vitro. Evidence of cell cycle blockade and apoptosis induction was obtained for the most effective compound 14c by means of flow cytometry, caspase-3 activity determination and microscopic techniques.

 Please wait while we load your content...
Please wait while we load your content...