Direct experimental observation of salt induced aspect ratio tunable PFPT silver-nanowire formation: SERS-based ppt level Hg2+ sensing from ground water†
Abstract
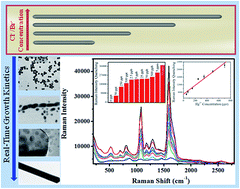
A plausible explanation based on real-time direct experimental observation has been put forward to explain the formation of common salt (NaF, NaCl, NaBr, and NaI) induced aspect ratio tunable Pentagonal Faceted Pyramidal Tipped (PFPT) silver-nanowires by considering the solubility product of the in situ generated silver halides, binding affinity of the added halides at the {111} facets on the growing front, and the free energy associated with the crystallographic planes (obtained from HRTEM study) as the driving forces. Aspect ratio dependent Raman activity was verified by using 4-mercaptobenzoic acid (4-MBA) as a Raman tag and due to the preferential binding of Hg2+ on the pyramidal tip of the silver-nanowire [i.e., {111} facet], as all the {100} facets are blocked by insoluble silver halides, controlled leaching of the nanowires offers the best-possible Raman platform for the ultra sensitive detection of Hg2+ contamination (up to 50 ppt) from ground water.

 Please wait while we load your content...
Please wait while we load your content...