A base promoted multigram synthesis of aminoisoxazoles: valuable building blocks for drug discovery and peptidomimetics†
Abstract
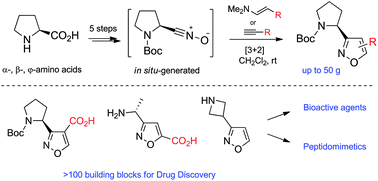
A practical multigram metal free synthesis of isoxazole-containing building blocks from commercially available amino acids was elaborated. The key reaction was a regioselective [3 + 2]-cycloaddition of in situ generated nitrile oxides with alkynes/enamines. The obtained building blocks were used in the preparation of bioactive compounds and peptidomimetics.

 Please wait while we load your content...
Please wait while we load your content...