Remediation of chromium(iii)-contaminated tannery effluents by using gallic acid-conjugated magnetite nanoparticles
Abstract
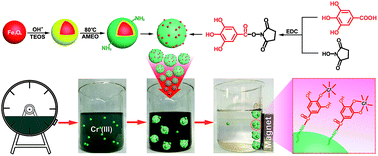
Potential ecological risks of chromium(III) contaminates in tannery effluents have evoked considerable discussion and re-examination over the future role of chrome tannage, which has been long considered as the foundation of the modern leather industry. Despite previous magnetite-supported adsorbents for chromium removal, few of them were specifically engineered to address trivalent chromium, as well as the composition complexity in tannery effluents. Herein, gallic acid, a natural triphenolic compound capable of coordination to chromium(III), was covalently conjugated onto engineered magnetite nanoparticles via 1-ethyl-3-(3-dimethylaminepropyl) carbodiimide hydrochloride/N-hydroxysuccinimide (EDC/NHS) chemistry, in an effort to design a magnetically separable nanoadsorbent applicable for remediating chromium(III)-contaminated tannery effluents. The structure of the nanoadsorbent was systematically characterized by multiple techniques, and the influence of pH value, adsorbent dose, temperature, and leather-related co-existing substances on its chromium(III) removal potency was investigated, respectively. Also, kinetics, equilibrium, and thermodynamics studies were conducted to decipher the mechanism by which chromium(III) cations were adsorbed. Finally, the feasibility of treating real tannery effluents that also contained high concentrations of sulfides, chlorides, ammonium nitrogen, total suspended solids, chemical oxygen demand (CODCr), and biochemical oxygen demand (BOD) by using the nanoadsorbent designed herein was explored. It was found that the chromium(III) removal percentage was approximately 95.2 ± 1.6%, and the exhausted nanoadsorbent could be conveniently separated from the effluents via a simple magnetic process. By chemical desorption, the nanoadsorbent was regenerable, and reusable for multiple cycles without a significantly compromised adsorption potency. According to these results, we aim at providing an efficient solution that may be a great addition to the ongoing fight against chromium(III) contamination in tannery effluents.

 Please wait while we load your content...
Please wait while we load your content...