Facile one-pot three-component synthesis of diverse 2,3-disubstituted isoindolin-1-ones using ZrO2 nanoparticles as a reusable dual acid–base solid support under solvent-free conditions†
Abstract
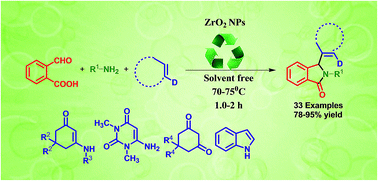
A facile one-pot three-component protocol for the synthesis of a series of multi-functionalized 2,3-disubstituted isoindolin-1-ones has been developed using ZrO2 nanoparticles as a dual acid–base solid support under solvent-free conditions. The surface of the ZrO2 nanoparticles, which is embedded with active hydroxyl groups, oxyanions and Zr4+ ions, efficiently catalyses the condensation of 2-carboxybenzaldehyde, aliphatic amines and a nucleophile (enamines/6-amino 1,3-dimethyluracil/1,3-cyclohexadiones/indole) to produce 2,3-disubstituted isoindolin-1-ones. Operational simplicity, reduced reaction time and temperature, elimination of solvent, high yields of products, wider substrate scope, utilization of ZrO2 nanoparticles as a solid support, and its nearly undiminished catalytic activity after repeated applications, are the key features of the present methodology.

 Please wait while we load your content...
Please wait while we load your content...