A highly efficient one-pot synthesis of indenopyridine-fused spirocyclic systems†
Abstract
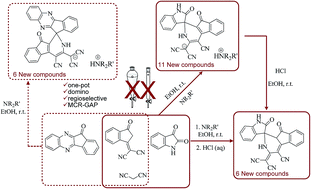
Structurally diverse spirooxindoles incorporating a medicinally privileged indenopyridine moiety have been synthesized regioselectively via multi-component reaction of 1,1-dicyanomethylene-3-indanone, isatins, and malononitrile in the presence of amines in ethanol as an environmentally friendly medium under mild conditions. This domino process includes a [5 + 1] hetrocyclization that occurs under metal-free conditions. The product is a spirooxindole-fused indenopyridine salt that was successfully neutralized by dilute hydrochloric acid. Replacement of isatins with indenoquinoxalines led to the production of dihydrospiro[indeno[1,2-b]quinoxaline-indenopyridine salts in good yields.

 Please wait while we load your content...
Please wait while we load your content...