The performance and mechanism for the catalytic oxidation of dibromomethane (CH2Br2) over Co3O4/TiO2 catalysts†
Abstract
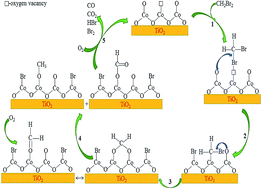
Brominated hydrocarbons are a typical pollutant in exhaust gas from the synthesis process of Purified Terephthalic Acid (PTA), and may cause various environmental problems once emitted into the atmosphere. Dibromomethane (DBM) was employed as the model compound in this study, and a series of Co3O4/TiO2 catalysts with various Co contents were prepared for the catalytic oxidation of DBM. The prepared catalysts were characterized by XRD, BET, SEM, TEM, XPS, H2-TPR and NH3-TPD. Among the prepared catalysts, CoTi-5 (5 wt% Co/TiO2) showed the highest catalytic activity, with T90 at about 346 °C, which was mainly attributed to the enrichment of well-dispersed Co3O4 and the high surface Co3+/Co2+ ratio, as it could provide more surface active sites and active oxygen species. The kinetic study showed that the reaction order of DBM was pseudo first-order and the reaction order of oxygen was approximately zero-order. A plausible DBM reaction mechanism over Co3O4/TiO2 catalysts was also proposed based on the results of in situ FTIR and the analysis of gas products by GC-MS. The reaction process started with the adsorption on surface oxygen vacancies, breakage of C–Br bonds and partial dissociation of C–H bonds with the formation of intermediate species, and then the intermediate species were further oxidized to form CO and CO2.

 Please wait while we load your content...
Please wait while we load your content...