Carbonyl scavenging and chemical chaperon like function of essential amino acids attenuates non-enzymatic glycation of albumin†
Abstract
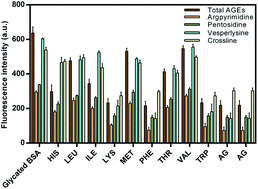
Besides being fundamental in the development of severe complications such as retinopathy, neuropathy and kidney disorders, non-enzymatic glycation also modulates the structure and function of many proteins such as albumin hemoglobin and collagen etc. Here we report the inhibitory activity of essential amino acids (EAA) against the formation of early and advanced glycation end products based on fluorescence, circular dichroism and molecular interaction studies. Assays were carried out to determine the formation of fructosamine, inhibition of fluorescent AGEs, protein bound carbonyl content, lysine modification and effect on glycation induced fibrillation based on thioflavin T (ThT) binding and confocal imaging. The protective effect of EAA on albumin conformation was determined based on the circular dichroism analysis. EAA exhibited differential effects on the non-enzymatic glycation induced structural modification of albumin. In addition to this, EEA also showed potent inhibitory activity towards in vitro goat lens protein. Tryptophan, phenylalanine and lysine showed the highest and valine showed the lowest antiglycation activity. The molecular interaction pattern of EAA as determined by computational methods revealed their affinity, binding site and the residues involved in binding.

 Please wait while we load your content...
Please wait while we load your content...