Microwave directed metal-free regiodivergent synthesis of 1,2-teraryls and study of supramolecular interactions†
Abstract
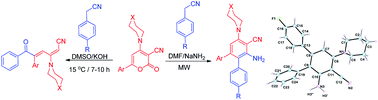
A microwave directed, simple and efficient synthesis of 3′-amino-5′-(sec.amino)-[1,1′:2′,1′′-teraryl]-4′-carbonitriles has been delineated through ring transformation reaction of 6-aryl-2-oxo-4-sec.amino-2H-pyran-3-carbonitriles by benzyl cyanide under basic conditions. Reaction of 6-aryl-4-sec.amino-2-oxo-2H-pyran-3-carbonitriles and benzyl cyanide in DMSO and KOH as a base at 15 °C provides (2E,4E)-5-aryl-6-oxo-6-phenyl-3-sec.amino-hexa-2,4-dienenitriles. Reaction of 6-phenyl-4-(piperidin-1-yl)-2-oxo-2H-pyran-3-carbonitrile and benzyl cyanide in DMF and sodamide at 100 °C provides 1,5-diphenyl-3-(piperidin-1-yl)cyclopenta-2,4-diene-1,2-dicarbonitrile. Use of microwave irradiation diverts the regioselectivity and changes the course of reaction and 1,2-teraryls was isolated. The structure of one of the compound was confirmed by single crystal X-ray and the nature of weak interactions in the compound has been addressed using ab initio, atoms in molecules (AIM) and Hirshfeld surface analysis.

 Please wait while we load your content...
Please wait while we load your content...