Anticancer drug delivery systems based on specific interactions between albumin and polyglycerol†
Abstract
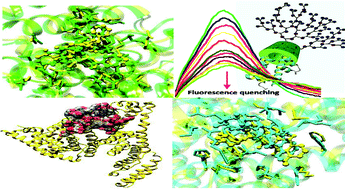
Here, we report for the first time a facile method for the preparation of novel drug delivery systems based on supramolecular interactions between β-cyclodextrin-polyglycerol conjugates (β-CD-g-PG) and human serum albumin (HSA). The results obtained by combined experimental/modeling studies showed that the main drivers to the formation of HSA/β-CD-g-PG supramolecular entities are host–guest interactions between β-CD-g-PG and the aromatic side-chains of HSA residues. Due to these interactions, HSA undergoes a confined yet fundamental conformational transition, leading to greater exposure of the hydrophobic protein domains available to hydrophobic drug binding. Next, the binding affinity and loading capacity of the HSA/β-CD-g-PG supramolecular nanovector for doxorubicin (DOX) and paclitaxel (PTX) were investigated. Both drug/HSA binding constants (Kb,HSA/DOX = 3.24 × 103 M−1 and Kb,HSA/PTX = 5.65 × 101 M−1) sensibly increased in the presence of β-CD-g-PG (Kb,HSA/β-CD-g-PG/DOX = 2.78 × 104 M−1 and Kb,HSA/β-CD-g-PG/PTX = 2.82 × 102 M−1). In line, both protein drug loadings increased by about 20% upon HSA/β-CD-g-PG interaction (80% and 71% for DOX and PTX, respectively) with respect to the loading capacity of the bare HSA (60% and 50%). Due to the improved loading capacity with minimal changes in the structure of HSA, this system is a promising vector for future cancer therapy.

 Please wait while we load your content...
Please wait while we load your content...