Recycling and synthesis of LiNi1/3Co1/3Mn1/3O2 from waste lithium ion batteries using d,l-malic acid
Abstract
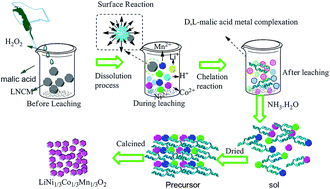
If waste LIBs are disposed of in landfill sites, soil contamination will ensue from leakage of the organic electrolyte, and the heavy metals ions contained in the batteries would pose a threat to the environment. A new process for recycling valuable metal ions from waste lithium-ion batteries (LIBs) is introduced herein. D,L-malic acid was used as both a leaching reagent and chelating agent. By adjusting the metal ion ratio and pH of leachate, a new cathode material of LiNi1/3Co1/3Mn1/3O2 for lithium ion batteries through a sol–gel process without other chelating reagents was synthesized. Electrochemical tests showed the initial charge and discharge capacity of the regenerated material to be 152.9 mA h g−1 and 147.2 mA h g−1 (2.75–4.25 V, 0.2C), respectively. The capacity retention at the 100th cycle remains 95.06% of the original value (2.75–4.25 V, 0.5C). Results indicated that the LiNi1/3Co1/3Mn1/3O2 produced from waste LIBs possessed good electrochemical properties.

 Please wait while we load your content...
Please wait while we load your content...