A combined H3PO4 activation and boron templating process for easy synthesis of highly porous, spherical activated carbons as a superior adsorbent for rhodamine B†
Abstract
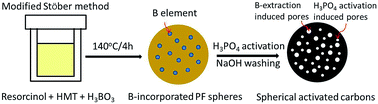
Spherical activated carbons (SACs) with a high surface area of 2729 m2 g−1 and a large total pore volume of 1.529 cm3 g−1 were easily prepared using phosphoric acid activation of polymeric precursor in which boron was embedded, followed by washing off of the boron phosphate formed in situ with a base solution. The reaction induced extraction of the pre-embedded boron from the carbon matrix which produced additional pores, and this greatly improved the textural properties of the SACs obtained. The SACs were tested as an adsorbent for bulky rhodamine B molecule, and the SACs exhibited an extremely high adsorption capability of up to 833 mg g−1 at ambient temperature at an adsorbent dosage of 1 g L−1. The adsorption isotherms and kinetics data showed a good fit with the Langmuir isotherm model and a pseudo-second-order kinetic model, respectively, and this is suggestive of the formation of a rhodamine B (RB) monolayer on the SACs possibly through chemical interactions such as ionic or covalent bonds.

 Please wait while we load your content...
Please wait while we load your content...