The Li–S battery: an investigation of redox shuttle and self-discharge behaviour with LiNO3-containing electrolytes†
Abstract
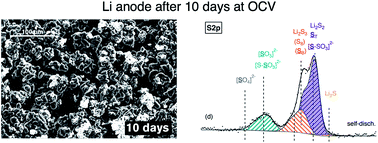
The polysulfide redox shuttle and self-discharge behaviour of lithium–sulfur (Li–S) cells containing the electrolyte additive LiNO3 has been thoroughly explored by a range of electrochemical and surface analysis techniques on simple Li–S (i.e., not specifically optimised to resist self-discharge) and symmetrical Li–Li cells. Despite the relatively effective passivation of the negative electrode by LiNO3, fully charged cells self-discharged a quarter of their capacity within 3 days, although in the short-term cells can be recharged without any noticeable capacity loss. The processes governing the rate and reversibility of self-discharge in these cells have been investigated and explained in terms of the reactions of polysulfides occurring at both electrodes during idle conditions.

 Please wait while we load your content...
Please wait while we load your content...