Ripening and recrystallization of NaCl nanocrystals in humid conditions†
Abstract
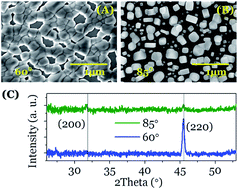
This study shows that Ostwald ripening, a universal mechanism responsible for the increase of crystal size during precipitation from solutions, can be meditated by ion diffusion through condensed monolayers of water that connect separated nanocrystals. In an environmental electron microscope we have observed “in situ” the time evolution of the number, shape, size and crystallographic texture of NaCl nanoparticles deposited by electron beam evaporation at oblique angles. Analysis of NaCl nanoparticles before and after water vapor condensation has evidenced that the size of nanocrystals is not the unique driving force inducing nanoparticle ripening and recrystallization, but the faceting of their crystalline habits and the amorphisation degree of the initially deposited nuclei also play important roles. These findings have implications for other crystallization and nucleation processes and can be of relevance for rock weathering and related phenomena.

 Please wait while we load your content...
Please wait while we load your content...