One-step process to form a nickel-based/carbon nanofoam composite supercapacitor electrode using Na2SO4 as an eco-friendly electrolyte
Abstract
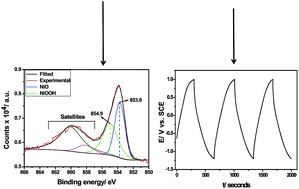
In this work, NiOx is anodically electrodeposited onto carbon nanofoam (CNF) to form a composite electrode devoted to supercapacitor applications. The use of NiSO4 as precursor in electrodeposition results in the formation of NiO and NiOOH species, as confirmed by XPS analysis, by means of a one-step anodic process. The presence of both NiO and NiOOH suggests the existence of pseudocapacitance, as observed in MnO2 and RuO2 materials. By employing Na2SO4, an eco-friendly electrolyte, the resulting composite delivers a specific capacitance of 150 F g−1 at 1 A g−1 considering the total mass of the electrode (deposit plus substrate). In addition, this composite electrode can operate in a very broad potential window, as high as 2.2 V, suggesting its application in high energy density electrochemical supercapacitors.


 Please wait while we load your content...
Please wait while we load your content...