Facile synthesis of well-shaped spinel LiNi0.5Mn1.5O4 nanoparticles as cathode materials for lithium ion batteries†
Abstract
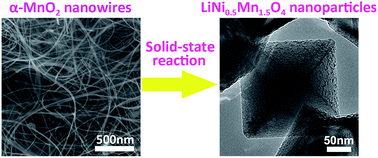
Spinel LiNi0.5Mn1.5O4 (LNMO) nanoparticles with well-defined polyhedral shapes and mean sizes of ca. 200 nm have been synthesized via a solid-state route using α-MnO2 nanowires as reaction precursors. A structural reorganization is believed to be responsible for the morphology evolution from tetragonal α-MnO2 nanowires to spinel LNMO nanoparticles. Galvanostatic charge–discharge measurements indicate the LNMO nanoparticles exhibit a high initial discharge capacity of 129 mA h g−1 with an 88% capacity retention over 100 cycles at 1C (147 mA h g−1), superior to those of LNMO nanorod counterparts (116 mA h g−1). The superior electrochemical performance of LNMO nanoparticles can be ascribed to their narrow particle size distribution, less particle aggregation, intimate interparticle contact, increased electrical conductivity and lithium ion insertion–extraction kinetics due to the existence of oxygen deficiency and exposed {111} crystal facets.

 Please wait while we load your content...
Please wait while we load your content...