A performance study on the electrocoating process with a CuZnAl nanocatalyst for a methanol steam reformer: the effect of time and voltage
Abstract
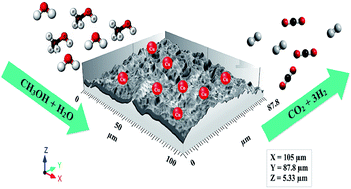
Coated microreactors provide an alternative strategy to enhance the performance of desired objects especially portable devices and in fuel cell applications. To successfully employ a catalyst in microreactors, study of coating methods is essential. In this paper, a high potential electrophoretic deposition technique was used to coat a CuZnAl catalyst on stainless steel. The prime aim of this examination is to determine the effects of the time and voltage of the coating on the catalyst performance. First, the CuZnAl catalyst was synthesized by a co-precipitation method to catalyze the methanol steam reforming. The powder was characterized by X-ray diffraction, ICP-OES and BET analyses. Second, based on the deposition time, methanol conversion was improved by an increase in the time of the coating to 4 minutes followed by a bit of a fall of both the conversion and deposition weight for a time of 5 minutes. Third, it was found that an increase in voltage from 60 to 140 V enhanced conversion while no deposition was achieved for higher voltages. The optimum conditions of 140 V and 4 minutes resulted in nearly full methanol conversion and a CO selectivity of 4.91% at 270 °C. Finally, the microreactor possessed a higher activity than that of a packed bed one. The coated layers were analyzed by field-emission scanning electron microscopy.

 Please wait while we load your content...
Please wait while we load your content...