Suzuki coupling for preparation of allenes – ligand effects and chirality transfer†
Abstract
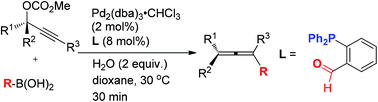
A general and efficient palladium-catalyzed coupling of propargylic carbonates with organoboronic acids has been developed by identifying a commercially available semi-labile bidentated o-(diphenylphosphino)benzaldehyde as the effective ligand affording allenes under a set of very mild reaction conditions. Many functional groups are tolerated in this reaction, and high chirality transfer has also been realized in the presence of water leading to a very rapid reaction for the synthesis of easily racemized 1,3-diarylallenes. A rationale has been proposed to demonstrate the role of the ligand.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers in 2016

 Please wait while we load your content...
Please wait while we load your content...