Molecular engineering of thiostrepton via single “base”-based mutagenesis to generate side ring-derived variants†
Abstract
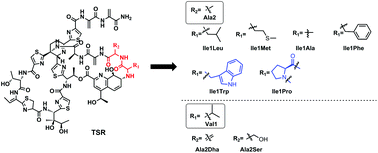
The quinaldic acid (QA) moiety in the side ring of thiostrepton (TSR), which can be modified regioselectively via precursor-directed mutational biosynthesis, was proven to be biologically relevant but tunable, affecting TSR's outstanding antibacterial activities. In this study, we sought to obtain TSR derivatives with varying amino acid residues connected to the QA moiety. The generation of these TSR derivatives relied on single “base”-based mutagenesis, and six new TSR-type compounds were obtained. Moreover, the simultaneous mutation of Ile1 and Ala2 in the TSR side ring resulted in a naturally occurring compound, siomycin (SIO), together with a new component, SIO-Dha2Ser. The anti-infection assays indicated that all of these new compounds could act as both antimicrobial agents and autophagy inducers, and these two kinds of activities can also be separated via regioselective modifications on the TSR side ring.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...