Abstract
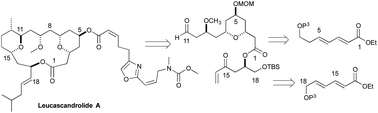
An asymmetric synthesis of the C1 to C11 and C14 to C18 fragments of the macrocyclic portion of the antibiotic Leucascandrolide A was achieved in 21 total steps from an achiral dienoate. The key 4-hydroxy-2,5-pyran portion of the natural product was established by oxy-Michael cyclization of a 5,7,9,11-tetraol intermediate, which in turn was established by an iterative asymmetric-hydration of dienoates. Alternative strategies for establishing the polyol stereochemistry were explored.
- This article is part of the themed collections: HOT articles in Organic Chemistry Frontiers in 2016 and Celebrating the 75th Birthday of Professor Barry Trost

 Please wait while we load your content...
Please wait while we load your content...