Synthesis and reactivity of alkoxy-activated cyclobutane-1,1-dicarboxylates
Abstract
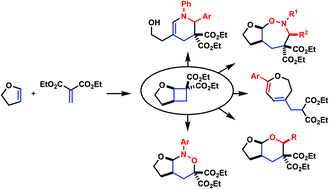
Intermolecular cycloaddition chemistry is unarguably one of the most appreciated strategies to bring complexity to products from simple starting materials. Generation of dipolar intermediates by exploitation of ring strain in carbocycles has become an efficient option. In this regard, donor–acceptor cyclopropanes have been extensively studied, but reports on extending similar synthetic transformations to the homologous cyclobutanes are comparatively limited. Recently, our group has become interested in application of alkoxy-activated cyclobutane-1,1-dicarboxylates (AACDs) in cycloaddition chemistry. This personal account discusses our contributions in this area with contextual examples from others.
- This article is part of the themed collections: Celebrating the 75th Birthday of Professor Barry Trost and 2016 Organic Chemistry Frontiers Review-type Articles

 Please wait while we load your content...
Please wait while we load your content...