Synthesis of unsymmetrical NCN′ and PCN pincer palladacycles and their catalytic evaluation compared with a related SCN pincer palladacycle†‡
Abstract
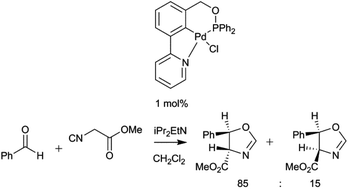
1-(3-(Pyridin-2-yl)phenyl)methanamine derivatives have been synthesized and underwent C–H bond activation to afford unsymmetrical NCN′ pincer palladacycles, which were characterised in the solid state. 2-Pyridinyl-phenol and -benzyl alcohols were then used as precursors to unsymmetrical PCN pincer palladacycles. Catalytic applications, where the palladacycle remains in the Pd(II) state, have been carried out and show good activity and selectivity.
- This article is part of the themed collection: Celebrating the 75th Birthday of Professor Barry Trost


 Please wait while we load your content...
Please wait while we load your content...