A general photoinduced electron transfer-directed chemoselective perfluoroalkylation of N,N-dialkylhydrazones†‡
Abstract
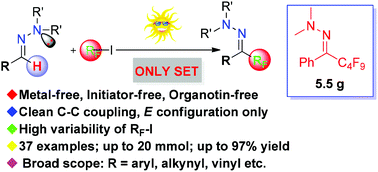
A selective, practical and general incorporation of fluoroalkyl groups into organic frameworks is of great interest for synthesis. Herein we report the first metal-free, initiator-free, and general photochemical perfluoroalkylation of a variety of N,N-dialkylhydrazones at room temperature in the absence of any external photocatalyst. It constitutes an important advance in perfluoroalkyl radical addition to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N π bonds for the synthesis of hydrazones instead of amines. Affordable and easily available perfluoroalkyl iodides serve as effective precursors. The excellent regio-, stereo- and chemoselectivity as well as the broad substrate scope make this a very promising synthetic tool.
N π bonds for the synthesis of hydrazones instead of amines. Affordable and easily available perfluoroalkyl iodides serve as effective precursors. The excellent regio-, stereo- and chemoselectivity as well as the broad substrate scope make this a very promising synthetic tool.
- This article is part of the themed collections: HOT articles in Organic Chemistry Frontiers in 2016 and Celebrating the 75th Birthday of Professor Barry Trost

 Please wait while we load your content...
Please wait while we load your content...