Light-driven highly efficient glycosylation reactions†
Abstract
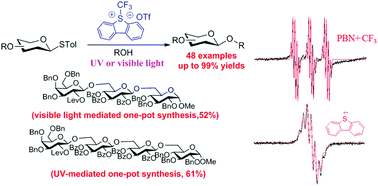
Glycosylation is unarguably the most important reaction in the field of glycochemistry. Photo-initiated reactions hold extraordinary potential in organic synthesis. Herein we report a novel light-driven strategy for the activation of thioglycosides via the merger of a CF3 radical pathway followed by the subsequent glycosylation with glycosyl acceptors. This protocol could efficiently activate thioglycosides, thereby greatly enhancing the substrate scope of reactions. In particular, the glycosylation reactions, which are completely inert under the existing photo-induced glycosylation conditions, proceeded smoothly in high yields. Many common protective groups were well tolerated under the coupling conditions. Both UV and visible light or even sunlight were used as the source of light for the reactions. The high efficiency of this light-driven glycosylation protocol was further highlighted by the rapid one-pot sequential assembly of oligosaccharides.

 Please wait while we load your content...
Please wait while we load your content...