Pd(ii)-catalyzed β-C–H arylation of O-methyl ketoximes with iodoarenes†
Abstract
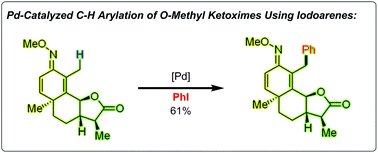
A Pd(II)-catalyzed selective β-arylation of O-methyl ketoximes was developed using iodoarenes as the coupling partners. This transformation has good functional group compatibility and can serve as a powerful synthetic tool for late-stage C–H arylation of complex compounds. Moreover, when employing 2-iodobenzoic acids as the substrates in this developed catalytic system, a type of unexpected five-membered lactones could be formed by tandem sp3 C–H arylation and oxygenation.
- This article is part of the themed collections: HOT articles in Organic Chemistry Frontiers in 2016 and 2015 Emerging Investigators by OCF

 Please wait while we load your content...
Please wait while we load your content...