Organocatalytic enantioselective Mukaiyama–Mannich reaction of fluorinated enol silyl ethers and cyclic N-sulfonyl ketimines†
Abstract
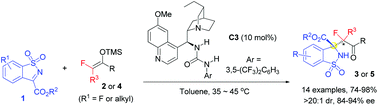
The first catalytic asymmetric Mukaiyama–Mannich reaction of fluorinated silyl enol ethers and ketimines is developed. Under the catalysis of hydroquinine derived bifunctional urea, cyclic N-sulfonyl ketimines readily react with fluorinated enol silyl ethers to afford benzosultam based Cα-tetrasubstituted α-amino acid derivatives featuring a fluoroalkyl group in high to excellent yields and stereoselectivities.

 Please wait while we load your content...
Please wait while we load your content...