Pd/norbornene-catalyzed sequential ortho-C–H alkylation and ipso-alkynylation: a 1,1-dimethyl-2-alkynol strategy†
Abstract
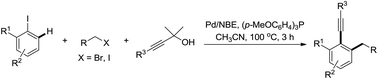
A palladium/norbornene-catalyzed ortho-C–H alkylation and ipso-alkynylation reaction for the synthesis of 2-alkyl-1-alkynyl arenes was reported. By the use of 1,1-dimethyl-2-alkynols as alkynyl reagents, the side O-alkylation reaction was mostly inhibited, and the reactivity of the three components matched well. As a result, A- and B-type side-products could be substantially inhibited and good to excellent yields were achieved.
- This article is part of the themed collections: HOT articles in Organic Chemistry Frontiers in 2016 and 2015 Emerging Investigators by OCF

 Please wait while we load your content...
Please wait while we load your content...