Magnesium and aluminum complexes bearing bis(5,6,7-trihydro quinolyl)-fused benzodiazepines for ε-caprolactone polymerization†
Abstract
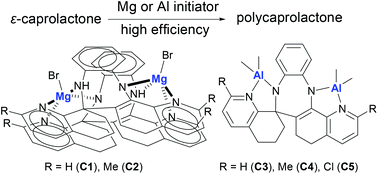
Substituted benzodiazepines of the general formula 2,2′-di(R)-5,6,6′,7′,8,13-hexahydro-5′H-spiro-benzo[2,3][1,4]diazepino[6,5-h]quinoline-7,8′-quinoline [R = H (L1), Me (L2), and Cl (L3)] were synthesized by the condensation reactions between 1,2-phenylenediamine and (2R)-5,6,7-trihydroquin-8-ones. Treatment of these compounds with two equivalents of C2H5MgBr or AlMe3 afforded the corresponding yellow magnesium complexes [R = H (C1) and Me (C2)], and the red aluminum complexes [R = H (C3), Me (C4) and Cl (C5)], respectively. The molecular structures of L1 and L3 and of complexes C1, C3 and C4 were determined by the single crystal X-ray diffraction study. L1 and L3 contain two 2R-substituted-5,6,7-trihydroquin-8-one groups fused with benzodiazepine through one-carbon or two-carbon atoms, respectively. The magnesium complex C1 displays a dinuclear bromomagnesium bridged by the ligand as the “pas de deux” arrangement, while aluminum complexes (C3 and C4) show bis(dimethylaluminum)benzodiazepines. In the presence of BnOH, the magnesium complexes showed efficient activities toward the ring-opening polymerization (ROP) of ε-caprolactone (ε-CL) with TOF up to 8100 h−1 at 60 °C; while the aluminum complexes also initiated high conversion of ε-CL by ROP.
The international collaboration between Professor Wen-Hua Sun at the Institute of Chemistry, Chinese Academy of Sciences (CAS), China, and Professor Pierre Braunstein at the Institute of Chemistry (UMR 7177 CNRS), University of Strasbourg, France, started in 2005 through the sharing of common scientific interests in the development of transition metal complexes as homogeneous catalysts. Prof. Sun was first a Visiting Professor at the University of Strasbourg in 2005, and Prof. Braunstein visited the Institute of Chemistry (CAS) giving a Molecular Science Forum Lecture in 2006. Supported by the Scientific Exchange Programme of the French Embassy in Beijing, Chinese graduate students from Sun's group moved into Braunstein's group as bilateral PhD candidates from 2007 to 2011. A PhD student from Sun's group also came to Strasbourg as a post-doctoral fellow. Furthermore, Profs. Sun and Braunstein successfully organized the “Sino-Franco-German Trilateral Symposium on Homogeneous Catalysis” in October 2007 in Beijing and in October 2010, the Symposium “Future of sciences, sciences for the future: Chemistry and its interfaces with biology and physics” was organized in Paris under the auspices of the CAS, French Academy of Sciences and German National Academy of Sciences Leopoldina. On this occasion, a special issue of C. R. Chim. was published (2011, issue 9). International exchanges and mutual visits are actively maintained between the two groups and Prof. Braunstein recently spent one month in China (mainly Beijing). Overall, Braunstein has supervised 16 Chinese PhD students and post-docs.
- This article is part of the themed collection: Sino-European Collaborators


 Please wait while we load your content...
Please wait while we load your content...