Magnetic relaxations in four-coordinate Dy(iii) complexes: effects of anionic surroundings and short Dy–O bonds†
Abstract
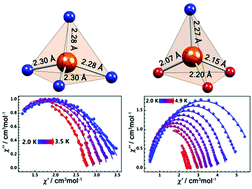
Two four-coordinate Dy(III) complexes, [LiTHF4][Dy(NPh2)4] (1) and DyLi(Ph3CO)3(NPh2)THF (2), with similar distorted tetrahedral geometries and a pure anionic coordination environment were synthesized under anaerobic conditions. Magnetic studies reveal that only 2 having a short Dy–O bond (2.07 Å) shows slow magnetic relaxation behaviour under zero dc field. Quantitative analyses of energy-levels and eigenstates using the program PHI reveal that the ground state doublets for both the complexes are mixed with low-lying states, though complex 2 shows much higher purity of ground state doublets. This study presumably indicates that the spherically coordinated anions with evenly distributed charges are adverse to the enhancement of the axial magnetic anisotropy of the Dy(III) ions, while the strong Dy–O bond may break such a high symmetry and hence promote the axial magnetic anisotropy of the Dy(III) ions.

 Please wait while we load your content...
Please wait while we load your content...