3d transition metal complexes with a julolidine–quinoline based ligand: structures, spectroscopy and optical properties†
Abstract
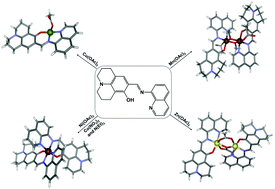
A Schiff base type ligand with the combination of the julolidine and the quinoline groups has been reported as a potential chemosensor in detecting the cobalt(II) ion among other heavy and transition metal ions in solution. However, no crystal structure of such a ligand with any metal ions has been reported. In this work, its complexation with 3d transition metal ions (Mn(II), Co(II), Ni(II), Cu(II) and Zn(II)) has been investigated with five new complexes being synthesised, and spectroscopically and structurally characterised. [Mn2L2(CH3OH)2(CH3COO)2]·CH3OH (1) {HL (C22H21N3O) = ((E)-9-((quinolin-8-ylimino)methyl)-1,2,3,5,6,7-hexahydropyrido[3,2,1-ij]quinolin-8-ol)} shows a dinuclear structure with two Mn : L : acetate (1 : 1 : 1) units bridged by two methanol molecules. [CoL2(NO3)]·CH3OH·H2O (2) and [NiL2]·H2O (3) exhibit mononuclear structures with a Co : L or Ni : L ratio of 1 : 2. [CuL(CH3COO)]·1/3CH3OH (4) demonstrates a mononuclear structure and the Cu ion has a square planar coordination polyhedron with a L ligand and a highly non-symmetrical acetate anion. [Zn2L2(CH3COO)2]·CH3OH (5) has two types of dinuclear units, both with two ZnL units bridged by two acetate anions but in three different bridging coordination modes. Their vibrational modes, absorption and photoluminescence properties have also been investigated.

 Please wait while we load your content...
Please wait while we load your content...