Internal Lewis pair enhanced H-bond donor: boronate-urea and tertiary amine co-catalysis in ring-opening polymerization†
Abstract
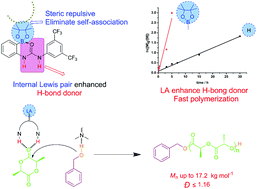
Co-catalysis with an H-bond donor (HBD) and base has been an important bifunctional catalytic system for polymerizations. The development of strong HBDs is a continuing quest in the design of more efficient H-bonding catalysts. Internal Lewis pair enhanced H-bond donors (LPHBDs) as strong HBDs have not been explored for polymerizations. Here we report that boronate-urea (BU) as a representative LPHBD, combined with (−)-sparteine as the base, promoted fast ring-opening polymerization (ROP) of L-lactide (LLA) at room temperature, which featured high conversions (up to 99%), predicted molecular weights (from 2.58 to 17.2 kg mol−1), and narrow dispersities (Đ ≤ 1.16). A bifunctional synergistic activation mechanism was proposed and supported by NMR titrations. The controlled/living nature of the ROP for LLA was confirmed by kinetics and chain extension experiments. 1H NMR, SEC, and MALDI-ToF MS analyses strongly indicated that the obtained PLAs were the designated ones. The successful synthesis of well-defined poly(trimethylene carbonate)-block-poly(L-lactide) verified that the catalytic ROP was of a controlled/living nature, and suggested that the LPHBD binary catalysis system is generally applicable. Taken together, we present a new protocol of internal Lewis pair enhanced H-bond (LPHBD) catalysis in ROP. Boronate-urea (BU) as a representative LPHBD is mild, tunable and it is more efficient than the common urea; it is nicely compatible with tertiary amines. BU combined with tertiary amines is a generally workable polymerization tool.


 Please wait while we load your content...
Please wait while we load your content...