Efficient synthesis of well-defined cyclic polystyrenes using anionic polymerization, silicon chloride linking chemistry and metathesis ring closure
Abstract
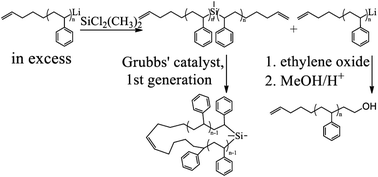
An efficient method for the synthesis of well-defined cyclic polystyrenes using anionic polymerization, silicon chloride linking chemistry, and metathesis ring closure has been developed. The macrocycle precursor, α,ω-bis(4-pentenyl)polystyrene, was formed by 4-pentenyllithium-initiated polymerization of styrene, coupling of α-pentenylpoly(styryl)lithium (PLi) with dimethyldichlorosilane to form α,ω-bis(4-pentenyl)polystyrene (Mn = 4600 g mol−1) and reaction of excess PLi with ethylene oxide to facilitate purification. Cyclization of the purified α,ω-bis(4-pentenyl)polystyrene was performed in dichloromethane under mild conditions using a Grubbs catalyst, bis(tricyclohexylphosphine)benzylidine ruthenium(IV) chloride, as a metathesis ring-closure agent. In contrast to prior work, no fractionation is required to obtain the pure product. Both the divinyl precursor and resulting macrocycle were characterized by SEC, MALDI-TOF mass spectrometry (MS) and NMR. The macrocycle was unambiguously distinguished from its precursor using the fragmentation patterns from tandem mass spectrometry (MS2) experiments. The results show that the macrocyclic precursor, α,ω-bis(4-pentenyl)polystyrene, was of high purity and that the cyclization was highly efficient.

 Please wait while we load your content...
Please wait while we load your content...