The influence of hydrogen bonding on radical chain-growth parameters for butyl methacrylate/2-hydroxyethyl acrylate solution copolymerization†
Abstract
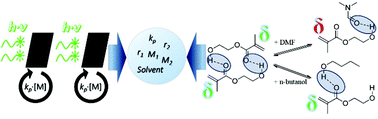
2-Hydroxyethyl acrylate (HEA), commonly introduced to control resin properties and introduce crosslinking sites, affects radical copolymerization kinetics through hydrogen-bonding, with the extent of the influence dependent upon solvent choice. The pulsed-laser polymerization technique has been applied to systematically investigate the influence of solvent choice on composition-averaged propagation rate coefficients (kcopp) and copolymer composition for HEA copolymerized with butyl methacrylate (BMA) in xylenes, dimethyl formamide (DMF), n-butanol (BuOH), methyl isobutyl ketone (MIBK) and butyl propionate. It is found that copolymer composition (as measured by proton NMR) has a greater dependency on solvent choice than propagation kinetics. HEA is incorporated into the BMA copolymer at higher relative rates than butyl acrylate (BA) in bulk, with the introduction of DMF and BuOH both reducing HEA incorporation rates. In contrast, the other solvents increase the relative reactivity of HEA, with reactivity ratios for the highest HEA incorporating case of BMA/HEA in xylenes found to be rBMA = 0.96 ± 0.05 and rHEA = 1.35 ± 0.14. Surprisingly, kcopp could be represented by the terminal model for polymerization in xylenes, MIBK and butyl propionate, but not for the bulk system.

 Please wait while we load your content...
Please wait while we load your content...