Cationic ring-opening polymerization of protected oxazolidine imines resulting in gradient copolymers of poly(2-oxazoline) and poly(urea)†
Abstract
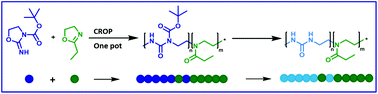
Poly(urea)s are a polymer class widely used in industry. Their utilization in biomedical applications is already described, however, the use of controlled polymerization methods instead of polycondensation approaches would allow a better control over the degree of polymerization and the dispersity of the resulting polymers, improving their suitability for this particular field of application. Cationic ring-opening polymerization (CROP) as a chain growth polymerization enables those requirements and, additionally, allows the copolymerization with 2-oxazolines, which are generally known for their biocompatibility. In this report, a Boc protected oxazolidine imine monomer is synthesized and polymerized in a homopolymerization, as well as in a copolymerization with 2-ethyl-2-oxazoline (EtOx) via CROP. The synthesized polymers were analyzed regarding their chemical and physical properties, using NMR, GC, MALDI-MS, SEC, TGA and DSC. Copolymerization kinetics revealed the formation of quasi-block copolymers, able to self-assemble in aqueous solution as indicated by DLS.

 Please wait while we load your content...
Please wait while we load your content...