Enhanced cytocompatibility and functional group content of poly(l-lysine) dendrimers by grafting with poly(oxazolines)†
Abstract
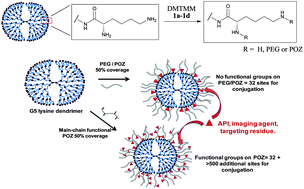
When considering the design of an advanced drug delivery system, a common desirable attribute is to have a prolonged residence time in blood circulation so that accumulation and localised payload release may occur at the site of interest (e.g. a tumour). Polyethylene glycol (PEG) has been a gold standard for fulfilling this requirement, and consequently has been well investigated as a material for surface modification of dendrimers. As an alternative, we have explored the use of polyoxazolines (POZ)s as materials for modifying the surface of a generation 5 L-lysine dendrimer and found that there was a significant improvement in the biocompatibility properties over the unmodified dendrimer. One particularly useful advantage of POZ over PEG lies in the main-chain pendant groups available that we were able to exploit to impart functionality. Modifying the POZ to have pendant carboxyl groups led to a novel modified dendrimer with significantly more sites for conjugation. With this, we have demonstrated a sixfold increase in the loading of coumarin (our model compound) when compared to a non-functional POZ equivalent.


 Please wait while we load your content...
Please wait while we load your content...