Highly selective and efficient chelating fiber functionalized by bis(2-pyridylmethyl)amino group for heavy metal ions†
Abstract
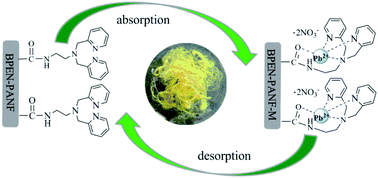
A bis(2-pyridylmethyl)amino group-modified polyacrylonitrile fiber (BPEN-PANF) was firstly prepared. The BPEN-PANF displays excellent selective complexation affinity in a 1 : 1 binding stoichiometry for the common toxic heavy metal ions, viz. Pb2+, Hg2+, Cd2+, Ag+, Zn2+, Cu2+, Ni2+, Co2+, and Cr3+. Meanwhile, it was significant that a much weaker binding ability was expressed for other light metal ions (Ca2+, Al3+, and Mg2+). Taking Pb2+ as an example, the maximum absorption capacity was as large as 303 mg g−1 calculated by the Langmuir isotherm model with an equilibrium time of 60 min. And the BPEN-PANF exhibits high absorption capacities for Pb2+ over a wide range of pH values from 3 to 11. Furthermore, it has an absorption limit of 4.76 ppb which is much lower than the drinking water standard of 10 ppb issued by the WHO. More positively, it can be easily recycled by filtration from the metal ion solutions and reused more than 20 times. In summary, the BPEN-PANF has the advantages of having inexpensive raw materials, simple preparation, high selectivity for heavy metal ions, and excellent recyclability and reusability, which surely indicates that the BPEN-PANF is a particularly useful material for rapid and simultaneous removal of heavy metal ions from polluted water.

 Please wait while we load your content...
Please wait while we load your content...