Maleimide-functionalized poly(2-ethyl-2-oxazoline): synthesis and reactivity†
Abstract
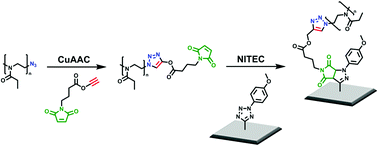
Poly(2-ethyl-2-oxazoline)s (PEtOx23 – Mn = 2300 g mol−1, Đ = 1.07; PEtOx46 – Mn = 4400 g mol−1, Đ = 1.06) end-functionalized with a maleimide moiety were prepared from azide-terminated PEtOxx-N3via copper-catalyzed azide–alkyne cycloaddition (CuAAC) with an alkyne-bearing maleimide (MI). The latter has been synthesized in a three-step procedure, including protection of the maleimide double bond prior to the modification of PEtOx. PEtOxx-MI was characterized by NMR (1H, 13C), SEC, FT-IR, and MALDI-ToF MS and, after deprotection of the maleimide, used in nitrile imine-mediated tetrazole-ene cycloaddition (NITEC) processes for covalent attachment to silicon surfaces in a grafting-to approach.

 Please wait while we load your content...
Please wait while we load your content...