Triphenylamine-based luminogens and fluorescent polyimides: effects of functional groups and substituents on photophysical behaviors†
Abstract
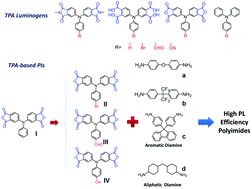
We prepared four series of triphenylamine (TPA)-based luminogens with various functional groups (diphthalic imide, tetracarboxylic acid, diphthalic anhydride, and pristine TPA) and substituted groups (–H, –Br, –CHO, and –CN), and corresponding fluorescent polyimides (PIs) were prepared from triarylamine-based dianhydride monomers with various aromatic and aliphatic diamine monomers for the investigation of their photophyscial behaviors. In the solution state, the introduction of strong electron acceptors such as formyl and cyano substituents in the luminogens induced strong emissions due to hybridized local and charge-transfer transitions (HLCTs). However, aggregated molecules containing these pendant electron-accepting groups resulted in quenching fluorescence behavior due to intermolecular interactions and energy transfers. Furthermore, the competition of aggregation-induced enhanced emission (AIEE) and aggregation-caused quenching (ACQ) effects was investigated and is discussed.

 Please wait while we load your content...
Please wait while we load your content...