Highly crystalline, low band-gap semiconducting polymers based on phenanthrodithiophene-benzothiadiazole for solar cells and transistors†‡
Abstract
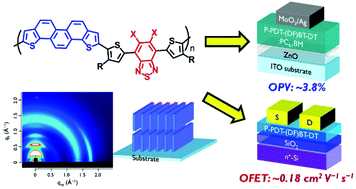
New PDT-based polymers were combined with two types of benzothiadiazole (BT) derivatives to improve their crystallinity and solar cell performance. These polymers show several advantages, including strong intermolecular interactions, deep HOMO energy levels, and a dense packing structure with a short π–π stacking distance of 3.5–3.6 Å. Combinations of PDT and BT units in polymers formed highly crystalline thin films with a long-range order, even in films blended with a fullerene derivative. This suggests that the introduction of optimal acceptor units may increase the regularity of the polymers, leading to effective π–π overlaps between the polymer backbones. However, although the present polymers also formed an appropriate phase separation structure in blended films, in fabricated solar cell devices they yielded low power conversion efficiencies (PCEs) not exceeding 3.8%. GI-WAXS analysis revealed that both polymers were present in a predominantly edge-on orientation. This unsuitable orientation for PSCs prevented efficient carrier transport and reduced charge collection efficiency, resulting in low Jsc and thus low PCE. On the other hand, these polymers also formed highly oriented edge-on structures on n+-Si/SiO2 substrates, which are suitable for high-performance field-effect transistors (FETs), and a fabricated FET device showed a hole mobility as high as 0.18 cm2 V−1 s−1.

 Please wait while we load your content...
Please wait while we load your content...