An aromatic/aliphatic polyester prepared via ring-opening polymerisation and its remarkably selective and cyclable depolymerisation to monomer†
Abstract
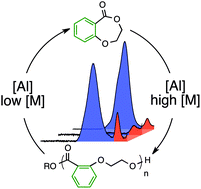
The ring-opening polymerisation of 2,3-dihydro-5H-1,4-benzodioxepin-5-one (2,3-DHB) with aluminium salen or organocatalysts gives polyester homopolymers and copolymers with L-lactide or rac-β-butyrolactone that contain both aromatic and aliphatic linkages, the first polymers with an aromatic ring in the backbone prepared by this key method. The same Al salen catalyst catalyses a remarkably selective depolymerisation to monomer under modified reaction conditions. The process may be cycled to repeatedly recycle polymer to monomer and maintain the polymer's low dispersity.
- This article is part of the themed collection: Open access articles from Polymer Chemistry

 Please wait while we load your content...
Please wait while we load your content...