Far beyond primary poly(vinylamine)s through free radical copolymerization and amide hydrolysis†
Abstract
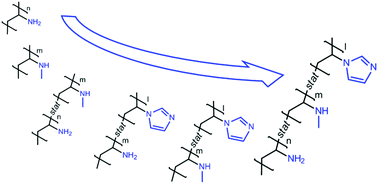
Due to their affinity for many supports, their pH responsiveness, metal binding capacity and polyelectrolyte complexation, poly(vinylamine) derivatives have attracted attention for many applications including coatings, water purification, or gas membrane separation. Nevertheless, most of them possess only pendant primary amines despite the possible benefits of incorporating different amino groups along the chain. In this work, a straightforward and scalable synthesis route towards polymers bearing primary and secondary amines, as well as imidazole groups, is reported. The general strategy relies on the radical copolymerization of different vinylamides and vinyl imidazoles followed by the hydrolysis of the resulting poly(vinylamide) derivatives. Binary and ternary free radical copolymerizations of N-vinylacetamide (NVA), N-methyl vinylacetamide (NMVA) and 1-vinylimidazole (VIm) were investigated and the reactivity ratios for each copolymerization system were determined. Thanks to these values a series of statistical copolymers with predictable composition and low deviation over the chain distribution could then be synthesized. Finally, the acidic hydrolysis of the acetamide functions towards the corresponding amine was performed and optimized. Copolymers containing various pendant amino groups and with low dispersity in the chain composition could be obtained, which opens new perspectives for the above mentioned applications.

 Please wait while we load your content...
Please wait while we load your content...