Optical properties and solvatofluorochromism of fluorene derivatives bearing S,S-dioxidized thiophene†
Abstract
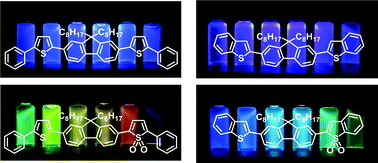
We synthesized fluorene derivatives having phenylthiophene (FPT) or benzothiophene (FBT), and their S,S-dioxidized compounds (FPTO2, FPTO4, FBTO2 and FBTO4), which are prepared by oxidation of the thiophene rings in FPT and FBT with m-chloroperoxybenzoic acid. FPT and FBT exhibited similar optical properties for absorption maximum wavelength, fluorescence maximum wavelength and fluorescence quantum yield. However, the absorption and fluorescence spectra of FPTO2 were largely shifted toward a longer wavelength in comparison with those of FPT, and their fluorescence quantum yields dramatically decreased with oxidation. In contrast, the absorption and fluorescence spectra and the fluorescence quantum yields of FBTO2 and FBTO4 were similar to those of FBT. Moreover, FPTO2 and FBTO2 showed strong solvatofluorochromism. Such solvent dependent properties are ascribed to the charge transfer character of the molecule.

 Please wait while we load your content...
Please wait while we load your content...