Intramolecular oxidative deselenization of acylselenoureas: a facile synthesis of benzoxazole amides and carbonic anhydrase inhibitors†
Abstract
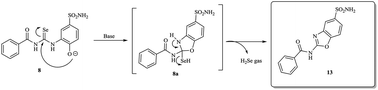
A mild, efficient and one pot procedure to access benzoxazoles using easily accessible acylselenoureas as starting materials has been discovered. Mechanistic studies revealed a pH dependent intramolecular oxidative deselenization, with ring closure due to an intramolecular nucleophilic attack of a phenoxide ion. All the benzoxazoles herein reported possessed a primary sulfonamide zinc binding group and showed effective inhibitory action on the enzymes, carbonic anhydrases.


 Please wait while we load your content...
Please wait while we load your content...