New one-pot synthesis of N-fused isoquinoline derivatives by palladium-catalyzed C–H arylation: potent inhibitors of nucleotide pyrophosphatase-1 and -3†
Abstract
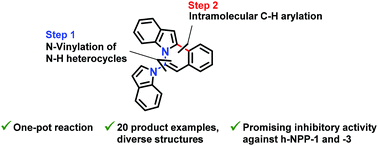
Various N-fused isoquinoline derivatives were synthesized using a new one-pot reaction of 1-bromo-2-(2,2-difluorovinyl)benzenes with N–H group containing heterocycles followed by intramolecular palladium-catalyzed C–H arylation. The method described gives convenient access to diverse structures of N-fused polycyclic isoquinolines. Sixteen of the synthesized compounds were screened as potential human nucleotide pyrophosphatase/phosphodiesterase 1 and 3 (h-NPP-1 and h-NPP-3) inhibitors. The most effective h-NPP-1 inhibitor showed an IC50 value as high as 0.36 ± 0.06 μM, whereas the most potent h-NPP-3 inhibitor posessed an inhibitory value of 0.48 ± 0.01 μM. Kinetic and molecular docking studies of both most effective inhibitors were carried out.

 Please wait while we load your content...
Please wait while we load your content...