Photochemical formation of quinone methides from peptides containing modified tyrosine†
Abstract
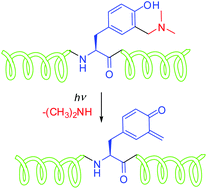
We have demonstrated that quinone methide (QM) precursors can be introduced in the peptide structure and used as photoswitchable units for peptide modifications. QM precursor 1 was prepared from protected tyrosine in the Mannich reaction, and further used as a building block in peptide synthesis. Moreover, peptides containing tyrosine can be transformed into a photoactivable QM precursor by the Mannich reaction which can afford monosubstituted derivatives 2 or bis-substituted derivatives 3. Photochemical reactivity of modified tyrosine 1 and dipeptides 2 and 3 was studied by preparative irradiation in CH3OH where photodeamination and photomethanolysis occur. QM precursors incorporated in peptides undergo photomethanolysis with quantum efficiency ΦR = 0.1–0.2, wherein the peptide backbone does not affect their photochemical reactivity. QMs formed from dipeptides were detected by laser flash photolysis (λmax ≈ 400 nm, τ = 100 μs–20 ms) and their reactivity with nucleophiles was studied. Consequently, QM precursors derived from tyrosine can be a part of the peptide backbone which can be transformed into QMs upon electronic excitation, leading to the reactions of peptides with different reagents. This proof of principle showing the ability to photochemically trigger peptide modifications and interactions with other molecules can have numerous applications in organic synthesis, materials science, biology and medicine.

 Please wait while we load your content...
Please wait while we load your content...